Views: 0 Author: Site Editor Publish Time: 2025-07-07 Origin: Site
Precipitated barium sulphate (BaSO₄) appears as a fine, white powder with exceptional brightness and purity. It stands out due to its uniform particle size, making it ideal for industries demanding high-quality fillers and pigments. Manufacturers rely on it for paints, plastics, rubber, and medical imaging because it delivers stability and consistent performance.
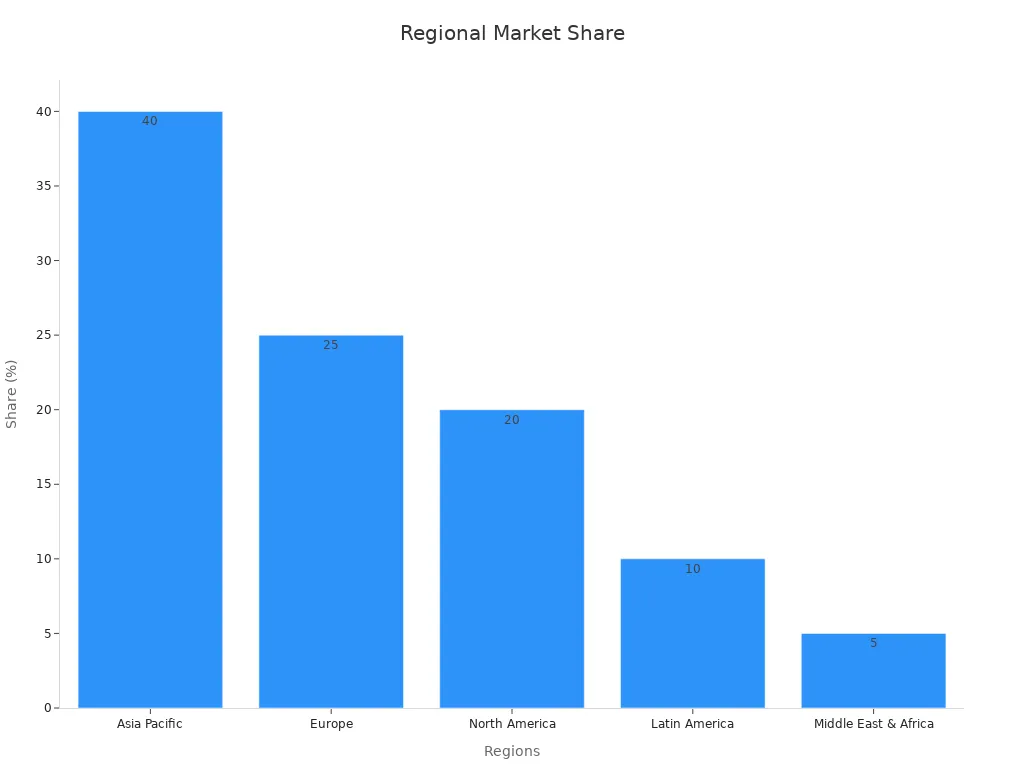
The global market for precipitated barium sulphate reached approximately USD 1.51 billion in 2023, with high demand in North America, Europe, and Asia Pacific.
Precipitated barium sulphate is a pure, fine white powder with uniform particle size, making it ideal for high-quality paints, plastics, rubber, and medical imaging.
Its chemical precipitation production method ensures high purity (around 99%) and controlled particle size, leading to consistent performance and safety.
The powder offers excellent whiteness, opacity, and chemical stability, resisting acids, alkalis, and weathering for long-lasting use.
It helps improve product quality while reducing costs, often replacing more expensive materials like titanium dioxide in paints.
Industries value precipitated barium sulphate for its safety, versatility, and reliability in demanding applications such as coatings, plastics, and medical imaging.
Precipitated barium sulphate stands out as a white, crystalline, and odorless powder. It shows remarkable purity, usually around 99%. The compound has a chemical formula of BaSO₄ and a molar mass of 233.39 g/mol. Its density reaches 4.49 g/cm³, and it melts at 1,580 °C. The powder remains insoluble in water and alcohol, but it dissolves in concentrated hot sulfuric acid. These properties make it stable and reliable for many industrial uses.
Property | Value |
|---|---|
Chemical formula | BaSO₄ |
Purity | ~99% |
Particle size (average) | ~0.7 µm |
Specific gravity | 2.7 |
Bulk density | 950 gm/lit |
Oil absorption | 19% |
pH of saturated solution | 8.2 |
Moisture content | 0.2% |
Appearance | White, crystalline, odorless |
Insolubility | Insoluble in water and alcohol |
Solubility | Soluble in concentrated hot sulfuric acid |
Melting point | 1,580 °C |
Boiling point | 1,600 °C (decomposes) |
Refractive index | 1.64 |
Scientists use several methods to measure the physical properties of precipitated barium sulphate. They apply sedimentation, air permeability, dye adsorption, and light-extinction techniques to determine specific surface area and particle size. Scanning electron microscopy (SEM) reveals that the particles are often spherical and about 1 µm in diameter. These measurements confirm the fine and uniform texture of the powder.
Note: The extremely low solubility of BaSO₄ in water (0.2448 mg/100 mL at 20 °C) ensures it remains stable in most environments.
Precipitated barium sulphate offers several advantages due to its unique features:
High purity: Gravimetric analysis confirms that the purity closely matches theoretical values, with deviations as low as 2.11%.
Consistent and controlled particle size: The manufacturing process allows precise control over particle size, which averages around 0.7 µm. This fine texture improves dispersion and performance in end products.
Morphological versatility: SEM images show that the particles can appear tablet-shaped, spherical, or flower-like, depending on processing conditions.
Chemical inertness: It resists acids, alkalis, and weathering, making it suitable for harsh environments.
Safety: Unlike other barium compounds, it does not dissolve in water, so it poses minimal toxicity risks.
High whiteness and opacity: The powder's bright white color and high refractive index enhance its value as a pigment and filler.
Researchers have found that the precipitation process can be adjusted to control purity and particle size. By changing stirring speed, temperature, and reactant concentrations, they can influence the shape and size of the crystals. For example:
Increasing supersaturation leads to smaller, less perfect crystals.
Adjusting the ratio of barium and sulfate ions changes particle morphology.
Using slow addition of reactants and heating improves purity and allows for larger crystals, which are easier to filter and wash.
These features make precipitated barium sulphate a preferred choice in industries that require high performance and reliability.
Manufacturers produce precipitated barium sulphate using a chemical precipitation method. This process involves mixing barium chloride and sulfuric acid in water. The reaction forms barium sulfate as a solid and hydrochloric acid as a byproduct. The chemical equation looks like this:
BaCl₂ (aq) + H₂SO₄ (aq) → BaSO₄ (s) + 2HCl (aq)
They control the reaction conditions to ensure the formation of fine, uniform particles. The process isolates primary precipitation from secondary effects, such as agglomeration. Scientists use advanced protocols to measure how quickly barium sulfate forms. These protocols help them understand how electrostatic and viscous forces affect particle interactions. The result is a process that consistently produces high-quality precipitated barium sulphate.
Laboratory tests confirm the efficiency of this method. Clean samples appear under scanning electron microscopy (SEM), showing pure and well-formed particles. Filtration tests also support the ability to create pure precipitates. Researchers observe how temperature and pressure affect the scaling tendency, which helps optimize the process for industrial use.
Note: The chemical precipitation method ensures low chlorine content in the final product, which is important for many applications.
The production process focuses on achieving high purity barium sulfate and a controlled particle size. Scientists use rapid mixing devices, such as Y-mixers, to influence nucleation rates and particle size distribution. Shock-Freeze Cryo-TEM imaging reveals particle sizes ranging from 62 nm to over 3,000 nm. Mixing speed and quenching methods play a key role in determining the final size and shape of the particles.
Parameter | Typical Value/Range |
|---|---|
Particle Size | 62 nm – 3,206 nm |
Purity | ~99% |
Chlorine Content | Very Low |
Low chlorine content remains a priority throughout the process. Synthetic barium sulfate produced this way meets strict industry standards. The ability to control particle size and purity makes precipitated barium sulphate suitable for demanding applications.
Precipitated barium sulphate stands out for its bright white color and exceptional opacity. Many industries value it as a white pigment because it delivers a clean, neutral shade. The fine, uniform particles scatter light efficiently. This property leads to increased opacity in paints, coatings, and plastics. Manufacturers often use it to improve opacity in products that require a solid, non-transparent finish.
Property | Benefit |
|---|---|
Brightness | Enhances visual appeal |
Opacity | Provides effective coverage |
Particle size | Promotes smooth dispersion |
The powder's high refractive index helps it act as an opaque white pigment. It covers surfaces well, even at low concentrations. Many paint and coating formulas rely on this material to achieve a consistent, bright finish. The result is a product that hides underlying colors and imperfections.
Tip: Using precipitated barium sulphate as a white pigment can reduce the need for more expensive materials like titanium dioxide.
Precipitated barium sulphate shows remarkable chemical stability. It resists acids, alkalis, and harsh weather conditions. The compound remains insoluble in water, so it does not leach or break down easily. This property makes it a safe choice for many applications.
Durability tests confirm its stability:
Accelerated aging laboratory tests show little change in structure.
Sulphate salt crystallisation tests reveal that barium sulphate forms a chemical shield. This shield reduces damage from sulphate crystallisation and volume changes.
Acid rain simulation tests demonstrate strong resistance to acidic environments.
Researchers use advanced techniques to study these effects. X-ray diffraction identifies stable phases in the material. Scanning electron microscopy with energy dispersive X-ray spectroscopy reveals a dense, uniform microstructure. Inductively coupled plasma-atomic emission spectroscopy confirms the chemical composition remains unchanged after exposure.
Precipitated barium sulphate also stands out for its non-toxicity. Unlike other barium compounds, it does not dissolve in water, so it poses minimal health risks. Many industries choose it for products that require safety and reliability. The compound's resistance to acids, alkalis, and weathering ensures long-lasting performance in outdoor and demanding environments.
Note: The chemical stability of precipitated barium sulphate helps protect building materials, paints, and plastics from environmental damage.
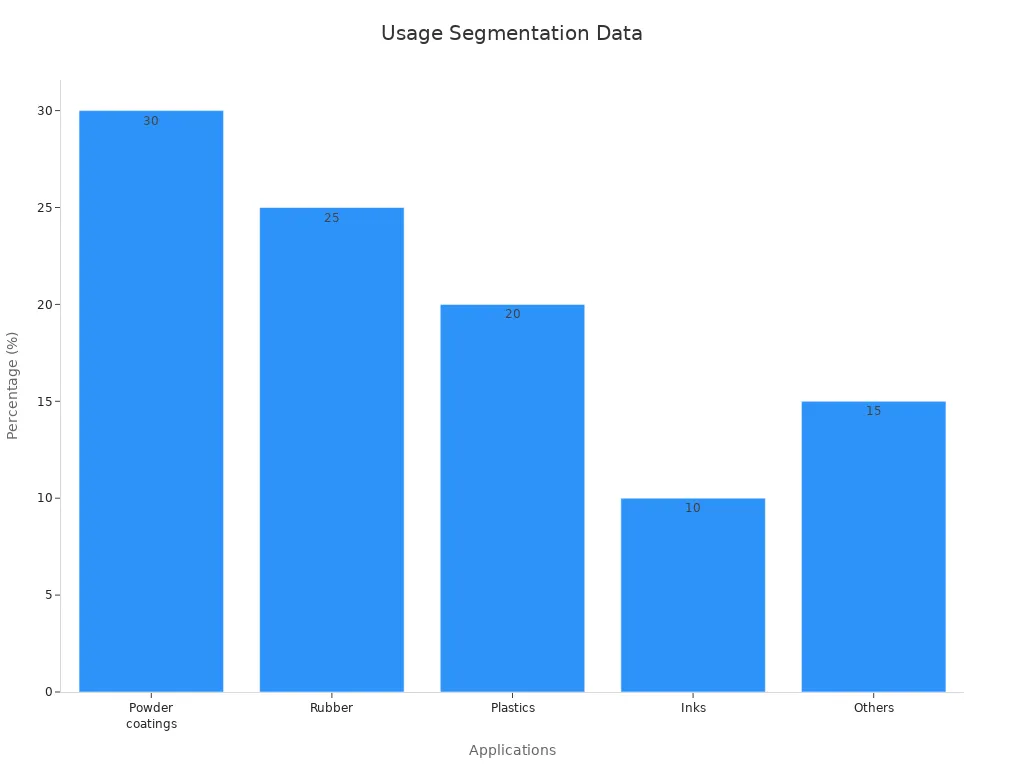
Precipitated barium sulphate plays a key role in paints and coatings. It acts as a filler and extender, improving opacity, whiteness, and gloss. Many manufacturers use it to reduce costs while maintaining high quality. Its fine particles scatter light, which increases coverage and hides surface flaws. The material resists weathering and chemicals, making paints last longer outdoors. Superfine grades boost brightness and flow, helping paints spread smoothly. Modified barium sulphate can even replace part of titanium dioxide without losing performance. It keeps paint glossy, easy to apply, and durable. These features make it popular in construction, automotive, and industrial coatings.
Tip: Using precipitated barium sulphate helps meet environmental rules for safer, more sustainable paints.
In plastics and rubber, precipitated barium sulphate serves as a universal diluent and reinforcing agent. It increases stiffness and impact resistance, making products stronger. The material also improves heat resistance and keeps shapes stable during use. Many producers add it to prevent clumping and improve how pigments spread. Nano-sized particles can raise tensile strength by up to 15% in some plastics. It also boosts printability and surface quality, which matters for 3D printing and specialty films. Its high refractive index gives plastics a brighter look and better light diffusion.
Increases mechanical strength and durability
Enhances heat and chemical resistance
Improves surface brightness and print quality
Precipitated barium sulphate stands out in medical imaging. Doctors use it as a radiopaque agent for X-rays and CT scans. It safely outlines organs because it does not dissolve in the body. The compound also finds use in inks, ceramics, and leather processing. In ceramics, it acts as a density modifier, giving products the right weight and feel. Many industries value its non-toxicity and stability for sensitive applications.
Metric/Aspect | Data/Insight |
|---|---|
Market Size 2024 | USD 1.96 billion |
Projected Market Size 2034 | USD 2.77 billion |
CAGR (2025-2034) | Approximately 3.5% |
Largest Market Share by Sector | Paint Industry (32.5% in 2023) |
Key Industrial Applications | Paints, coatings, plastics, pharmaceuticals, healthcare, oil & gas, automotive |
These trends show steady growth in demand for precipitated barium sulphate across many industries. Its versatility and safety drive its expanding use worldwide.
Natural barium sulphate comes from mining baryte ore. Workers extract it from the earth, then crush and grind it into barite powder. This process uses simple mechanical steps. It often leaves impurities in the final product. Most natural barium sulphate contains less than 85% purity. The particle size stays coarse, usually around 300 mesh. Producers use basic beneficiation to remove some unwanted minerals, but the result still shows lower whiteness and more variation in quality.
Precipitated barium sulphate takes a different path. Chemists create it in controlled environments using chemical reactions. They mix barium chloride and sulfuric acid to form a fine, white powder. This method produces up to 99% purity. The particle size becomes much finer, often above 10,000 mesh. The process allows for precise control over particle distribution and shape. It also removes most impurities, giving the powder high whiteness and uniformity.
Parameter | Natural Barium Sulphate | Precipitated Barium Sulphate |
|---|---|---|
Purity (%) | Generally less than 85% due to impurities | Up to 99% after chemical processing |
Particle Size (mesh) | Mainly around 300 mesh | Generally above 10,000 mesh (much finer) |
Particle Fineness (AIM) | Lower AIM value (coarser) | Higher AIM value (finer particles) |
Typical Applications | General paints, plastics (lower purity needs) | High-end coatings, automotive coatings, electronic inks, engineering plastics |
Production Process | Simple mining and processing | Complex chemical precipitation and purification |
Cost | Lower due to simpler process | Higher due to complex processing and energy consumption |
Natural barium sulphate finds its main use in bulk industries. Oil and gas companies use it in drilling fluids because of its high density and inertness. It also appears in general paints and plastics where purity does not matter as much. The lower cost makes it attractive for large-scale, cost-sensitive projects. However, the presence of impurities and larger particle size limit its use in advanced applications.
Precipitated barium sulphate serves high-end markets. Manufacturers choose it for powder coatings, automotive paints, advanced plastics, and electronic inks. Its high purity and fine particle size deliver better performance, brightness, and stability. Pharmaceutical and healthcare industries rely on it for safety and consistency. The controlled production process ensures each batch meets strict standards.
Aspect | Precipitated Barium Sulphate | Natural Barium Sulphate |
|---|---|---|
Source | Chemically synthesized | Derived from baryte ore |
Purity | High purity, low impurities | Contains impurities, requires beneficiation |
Particle Size Distribution | Controlled and uniform | Less controlled |
Whiteness | High whiteness | Lower whiteness |
Solubility | Low solubility | Higher solubility |
Primary Applications | High-end: paints, coatings, pharmaceuticals, advanced plastics, rubber | Bulk industrial: oil & gas drilling fluids |
Market Value & Growth | Higher market value; USD 2.5 billion in 2023; CAGR 5.5% (2023-2030) | Commodity-driven, lower growth |
Regional Abundance | Asia-Pacific dominance | Abundant in China, India, Morocco |
Key Industry Requirements | Consistency, performance, environmental benefits | Cost-sensitive, density and inertness critical |
Emerging Trends | Nanostructured forms, environmentally friendly production | N/A |
Natural barium sulphate remains important for industries that need large amounts of material at a lower price. Precipitated barium sulphate leads in sectors that demand high performance, purity, and environmental safety. Both types play key roles, but their differences shape where and how companies use them.
Precipitated barium sulphate offers unique benefits. It provides high purity, safety, and versatility. Many industries rely on it for consistent quality and performance.
Paints, plastics, and medical imaging use it every day.
Advanced applications demand its fine particle size and stability.
People encounter this material in products they use daily. Its expanding role highlights its growing importance in modern technology and manufacturing.
Precipitated barium sulphate has higher purity and finer particles. It comes from a controlled chemical process. Natural barium sulphate comes from mined ore and often contains impurities. Industries choose the precipitated form for products that need brightness and consistency.
Yes. Doctors use it as a contrast agent in X-rays and CT scans. It does not dissolve in the body, so it passes through safely. Its non-toxic nature makes it a preferred choice for medical applications.
Manufacturers often use it to partially replace titanium dioxide. It improves opacity and brightness while lowering costs. The combination helps paints cover surfaces well and look bright.
Industry | Main Use |
|---|---|
Paints/Coatings | Filler, pigment |
Plastics/Rubber | Reinforcement, brightness |
Medical | Imaging agent |
These industries rely on its purity, whiteness, and stability for high-quality products.
Copyright 2024 GUANGZHOU TIPTOP NEW MATERIAL CO., LTD. Sitemap

