Views: 0 Author: Site Editor Publish Time: 2025-07-10 Origin: Site
Natural barium sulphate comes from barite ore and offers cost benefits, while precipitated barium sulphate provides higher purity and finer particles for demanding applications. Many industries in 2025 must choose between these two types. Growth in Asia-Pacific and strict regulations in Europe drive the need for careful selection.
Key market highlights:
North America sees growth in paints, coatings, and plastics.
Europe favors high-purity, eco-friendly options.
Asia-Pacific expands rapidly due to industrialization.
Price volatility and regulations challenge manufacturers.
Technological advances support sustainable, pure products.
Feature | Natural Barium Sulphate | Precipitated Barium Sulphate |
|---|---|---|
Purity | Moderate | High |
Particle Size | Larger | Finer |
Cost | Lower | Higher |
Typical Applications | Drilling, plastics | Coatings, medical, paper |
Natural barium sulphate is cost-effective and suits bulk uses like drilling and plastics, while precipitated barium sulphate offers high purity and fine particles for advanced applications like medical imaging and coatings.
Production of natural barium sulphate involves mining and physical separation, leading to moderate purity; precipitated barium sulphate is made chemically, allowing precise control of purity and particle size.
Smaller and uniform particle sizes in precipitated barium sulphate improve product strength, appearance, and performance, especially in sensitive industries like healthcare.
Price differences reflect production complexity: natural barium sulphate is cheaper due to simpler processing, while precipitated barium sulphate costs more but delivers higher quality.
Choosing the right type depends on application needs, budget, and regulations; companies should match barium sulphate grades to their specific industry requirements for best results.
When comparing natural barium sulphate and precipitated barium sulphate, several important differences stand out. Natural barium sulphate comes from barite ore. It usually contains more impurities. Precipitated barium sulphate forms through a chemical process. This method creates a product with higher purity and finer particles.
Aspect | Natural Barium Sulphate | Precipitated Barium Sulphate |
|---|---|---|
Source | Barite ore | Chemical precipitation |
Purity | Moderate | High |
Particle Size | Larger, less uniform | Finer, very uniform |
Cost | Lower | Higher |
Main Uses | Drilling fluids, plastics | Paints, coatings, medical imaging |
Note: Precipitated barium sulphate offers better control over purity and particle size. Natural barium sulphate provides a cost-effective option for less demanding uses.
Natural barium sulphate gives industries a low-cost filler. It works well in drilling fluids and some plastics. Many companies choose it when high purity is not required.
Precipitated barium sulphate stands out for its high purity and fine, consistent particles. It fits best in paints, coatings, and medical products. These applications need strict quality and performance.
Natural barium sulfate also supports industries that need bulk material at a lower price. It remains a popular choice in regions where cost matters most.
Precipitated barium sulphate helps manufacturers meet strict regulations. It supports advanced technologies and eco-friendly products.
This quick comparison helps buyers and engineers decide which type fits their needs. They can weigh cost, purity, and performance before making a choice.
Natural barium sulphate comes from barite ore, a dense mineral valued for its high specific gravity. The production process starts with mining, either from open-pit or underground sources. Workers crush and mill the ore to reduce its size. They then use manual separation to pick out high-grade barite blocks based on color and glossiness. Gravity separation follows, using machines like jigs and table concentrators to concentrate the barite. Magnetic separation removes iron-rich impurities, while flotation helps separate barite from more complex ores. Some facilities use leaching to remove colored impurities, such as iron or manganese, which can affect whiteness. Calcination removes moisture and oxidizable impurities, improving both whiteness and purity.
These production processes help natural barium sulphate meet industry standards for drilling fluids, plastics, and paints. Over time, technological advances have made extraction and processing more efficient. Environmental regulations now encourage sustainable practices, making natural barium sulfate a preferred choice for eco-friendly products. The market continues to grow, especially in regions focused on recyclable plastics and low-VOC coatings.
Note: Natural barium sulphate holds a strong market share due to its affordability, non-toxic nature, and established production methods.
Precipitated barium sulphate uses a chemical reaction to achieve high purity and fine particle size. The production process involves mixing barium salts, such as barium chloride, with sulfate sources like sodium sulfate in a controlled reactor. This reaction forms barium sulfate as a solid precipitate. Engineers control the process by adjusting mixing intensity, reactor size, and residence time. These factors help achieve a uniform particle size distribution, which is important for applications in coatings, medical imaging, and electronics.
Process control relies on monitoring particle size and mixing conditions. Advanced techniques, such as Kalman filters, estimate particle size when direct measurement is difficult. Reactor design and hydrodynamics also play a role in the final product’s quality. Innovations in the production processes allow manufacturers to tailor precipitated barium sulphate for specific uses, improving both efficiency and sustainability. The demand for high purity barium sulfate production continues to rise, especially in industries that require strict quality standards.
Process Step | Natural Barium Sulphate | Precipitated Barium Sulphate |
|---|---|---|
Source | Barite ore | Chemical reaction |
Main Processing Methods | Mining, crushing, separation | Precipitation in reactors |
Purity Control | Physical beneficiation | Process control, monitoring |
Particle Size Control | Milling, separation | Reactor design, mixing intensity |
Natural barium sulphate comes from barite ore. It often contains impurities like iron, silica, and calcium. These impurities can vary from batch to batch. The barium sulfate content in natural sources usually ranges from 90% to 96%. Processing methods such as gravity separation and flotation help remove some unwanted materials. However, it rarely reaches the high purity needed for sensitive applications. Industries use it when moderate purity is acceptable. It works well in drilling fluids, plastics, and some paints. Consistency can change due to differences in ore quality and processing.
Precipitated barium sulphate forms through a controlled chemical reaction. This process allows manufacturers to achieve high purity, often above 98%. The barium sulfate content stays consistent from batch to batch. Engineers can control particle size and remove almost all impurities. This high purity makes it suitable for demanding uses. It meets strict standards in medical imaging, pharmaceuticals, and advanced coatings. The uniform composition ensures reliable performance in every application.
Purity and barium sulfate content play a key role in how industries use these materials. Sensitive sectors like healthcare and pharmaceuticals require high purity and consistent composition. Precipitated barium sulphate meets these needs. It provides the safety and performance needed for medical imaging and drug delivery.
X-ray diffraction (XRD) checks the crystalline structure and phase purity of barium sulfate nanoparticles. This affects how well they work in medical imaging.
Dynamic light scattering (DLS) measures particle size and stability. These properties matter for how nanoparticles behave in the body.
High purity and controlled particle size improve the effectiveness of barium sulfate as a contrast agent in CT scans. They also help in drug delivery by improving solubility and targeting.
Metric / Property | Observed Effect with Increased Barium Content |
|---|---|
Relative Density (%) | Increased from ~62.7% (control) to ~94.3% (5 mol% Ba) |
Mechanical Strength (GPa) | Increased from 0.86 GPa to 5.06 GPa with higher Ba2+ concentration |
Bioactivity (Apatite Formation) | Enhanced apatite formation after 21 days in simulated body fluid (SBF) |
Cell Viability (%) | Alginate microbeads crosslinked with Ba2+ showed up to 90% viability after 9 days |
Biocompatibility (Hemolysis %) | Improved biocompatibility with increasing barium oxide content in bioactive glasses |
Cell Proliferation (fold increase) | Fibroblasts and U937 cells showed 21x and 6.6x proliferation after 7 days |
Osteoblast Activity | Significant calcium and alkaline phosphatase deposition, 13.5x increase after 21 days |
In Vivo Biocompatibility | No inflammation or negative effects observed in bone repair systems with barium after 6 weeks and 2 years |
These results show that higher barium sulfate content and high purity improve mechanical strength, bioactivity, and safety. Medical imaging and drug delivery systems benefit from these properties. Consistent purity also helps in marine applications, where stability and longevity matter. Organic matter can protect barium sulfate crystals, making them last longer in seawater.
Note: Industries that need strict quality, such as healthcare and advanced manufacturing, choose precipitated barium sulphate for its high purity and reliable barium sulfate content. Natural barium sulphate remains useful for less sensitive applications where moderate purity is enough.
Natural barium sulphate, often called barite, goes through crushing, washing, drying, and micronization. These steps reduce its particle size from about 25 micrometers down to a range of 2–10 micrometers. This smaller size helps it disperse better in products like plastics and paints. The particle size distribution often depends on processing conditions, such as pH and the use of additives. At a neutral pH, particles can be less than 1 micrometer and show a uniform distribution. When the pH drops, particles become larger and less uniform. The table below shows how different conditions affect particle size and uniformity:
Condition | Particle Size Characteristics | PSD Curve Features | Notes on Uniformity and Morphology |
|---|---|---|---|
Natural pH | < 1 μm | Single peak | Uniform, well dispersed |
pH = 3 | Larger, less uniform | Double peak | Prone to clumping |
pH = 7 | Uniform | Side peak | Good dispersibility |
pH = 9 | Uniform | Single peak | Less symmetrical than at pH 12 |
pH = 12 | Narrow, symmetrical | Single peak | Most uniform, best with MGDA-3Na additive |
Precipitated barium sulphate forms through a chemical process that allows for very fine and uniform particles. Manufacturers use precise controls to achieve particle sizes often below 1 micrometer. They measure these fine particles using sensitive balances and filtration techniques. The process removes static charges and ensures accurate weighing, which helps keep the particle size consistent. Scientists use models like the Weibull distribution to describe how these particles spread out in size. This high level of control means precipitated barium sulphate can meet strict requirements for medical and high-tech uses.
Particle size plays a big role in how barium sulphate performs in different products. Smaller, more uniform particles improve strength, durability, and appearance. Several tests show these effects:
Mercury porosimetry reveals that as particle size increases, pore size in materials also grows, which can weaken the product.
Mechanical strength tests show that larger particles do not bond as well, making materials more likely to break.
Water absorption tests find that larger particles increase absorption, while finer particles help keep products strong and less porous.
In pharmaceuticals, smaller particles dissolve more evenly, which improves how medicines work in the body.
Tip: Choosing the right particle size can boost product quality, whether for paints, plastics, or medical uses. Finer particles often lead to better performance and reliability.
Natural barium sulphate finds use in many industries due to its chemical stability and cost-effectiveness. Companies often select it for natural barium sulfate applications where moderate purity is acceptable and bulk material is needed.
Powder coatings use it as a rheology modifier, density controller, opacity agent, thermal stabilizer, and abrasion resistance enhancer.
Oil and gas drilling operations rely on it as a weighting agent in drilling mud. It helps maintain borehole stability and supports efficient deep well drilling.
Medical imaging uses its high density and X-ray absorption properties. It acts as a radiological contrast agent for certain diagnostic procedures.
Cosmetics and personal care products benefit from its environmental friendliness and chemical stability.
Industrial processes address flow assurance challenges by studying the formation and inhibition of barium sulphate scale in oilfield equipment.
Research using synchrotron X-ray diffraction explores how barium sulphate scale forms on stainless steel in oilfield equipment. Scientists identify crystal growth patterns and test chemical inhibitors. These studies help maintain equipment functionality and reduce maintenance costs in the oil and gas sector.
Application Area | Function/Benefit |
|---|---|
Powder Coatings | Improves flow, density, opacity, and abrasion resistance |
Oil & Gas Drilling | Acts as a weighting agent for drilling fluids |
Medical Imaging | Serves as a radiological contrast agent |
Cosmetics/Personal Care | Adds stability and safety |
Industrial Equipment | Addresses scale formation and flow assurance |
Precipitated barium sulphate offers high purity and fine, uniform particles. These qualities make it ideal for high-end applications that demand strict quality and performance.
Paints and coatings benefit from its excellent whiteness, low oil absorption, and chemical inertness. It improves product quality and durability.
Plastics and rubber industries use it as a cost-effective filler. It enhances mechanical strength and surface finish.
Modified and nanometer grades provide improved dispersibility, higher purity, and better particle size distribution. These grades suit electronics, healthcare, and aerospace.
Pharmaceuticals use it as a contrast agent for X-ray imaging due to its safety and consistency.
Construction materials, such as cement and advanced composites, gain from its chemical stability and non-toxic nature.
Oil and gas drilling fluids also use it as a weighting agent, especially where high purity is required.
Verified industry data confirm its versatility and growing demand. The market for precipitated barium sulphate reached USD 1.2 billion in 2024 and is projected to hit USD 2.5 billion by 2033, with a CAGR of 9.5%. The Asia-Pacific region leads in growth, driven by industrialization and demand for premium, eco-friendly products.
Aspect | Details |
|---|---|
Market Revenue (2024) | USD 1.2 Billion |
Projected Revenue (2033) | USD 2.5 Billion |
CAGR (2026-2033) | 9.5% |
Key Benefits | High density, chemical stability, non-toxic, excellent whiteness, low oil absorption |
Primary Applications | Filler in paints, coatings, plastics, rubber; weighting agent in oil & gas drilling fluids |
Market Drivers | Demand for high-quality pigments/fillers in paints and coatings; growth in automotive, construction sectors |
Market Opportunities | Lightweight materials for automotive/aerospace; eco-friendly products; advanced composites |
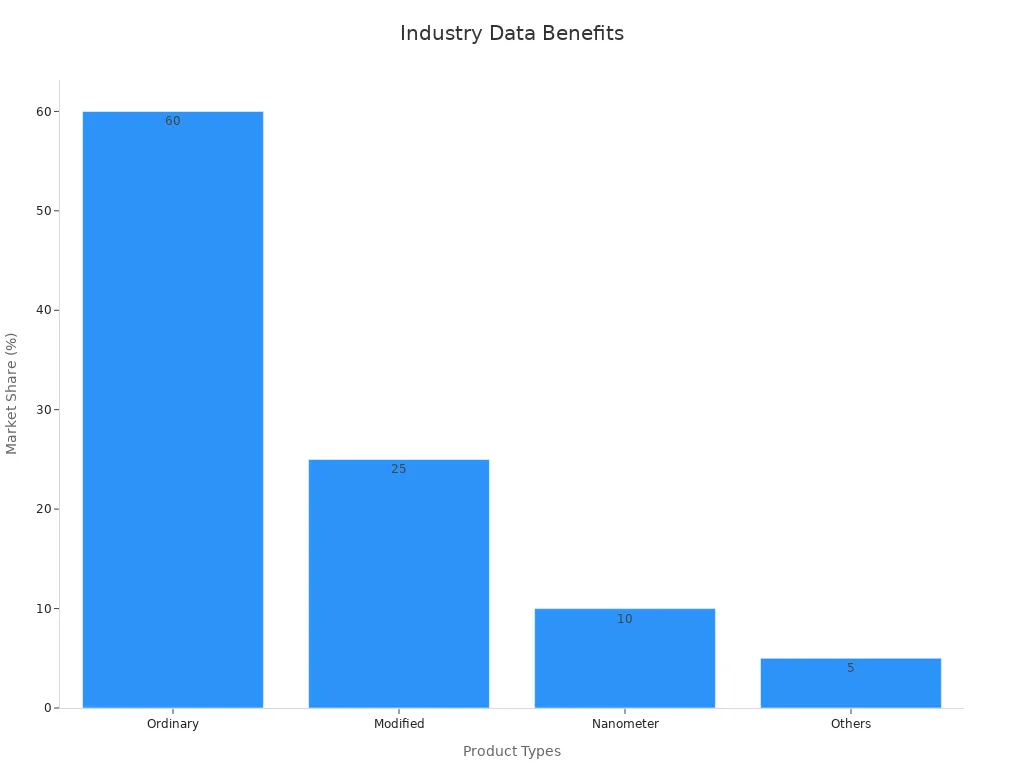
Market Share by Type (2023) | Ordinary: 60%, Modified: 25%, Nanometer: 10%, Others: 5% |
Market Share by Application | Coatings: 40%, Rubber: 30%, Plastics: 20% |
Regional Growth | Asia-Pacific leading and fastest-growing market |
Additional Notes | Modified and nanometer variants offer improved purity, dispersibility, particle size, mechanical strength |
Industries select between natural and precipitated barium sulphate based on performance needs, cost, and regulatory requirements. Market analysis shows both types play important roles in downstream applications.
The Fortune Business Insights report highlights extensive use in X-ray contrast agents, radiation shielding materials, well-drilling fluids, paints, pigments, plastics, and catalyst supports.
Precipitated barium sulphate holds a 42.6% revenue share in 2023, favored for high brightness and opacity in paints, plastics, and paper coatings.
Natural barium sulphate accounts for 34.1% revenue share, preferred in cost-sensitive uses such as fillers in plastics, rubber, and construction.
The largest product segment is powder form, used in over 60% of applications. Granules grow fastest in drilling fluids, while liquid forms serve medical imaging.
End-use industries include automotive (brake linings, clutches), construction (cement, paints), electronics (dielectric material, fillers), healthcare (X-ray contrast agent), packaging, printing, and textiles.
Segment Category | Details |
|---|---|
Market Share by Grade | Precipitated: 42.6% (high brightness/opacity); Natural: 34.1% (cost-sensitive fillers) |
Applications | Oil & Gas drilling fluids, paints & coatings, paper & pulp, plastics, glass, medical imaging, printing inks, rubber, cosmetics, fertilizers |
Product Types | Powder (>60%), Granule (fastest growth), Liquid (medical imaging) |
End-Use Industries | Automotive, Construction, Electronics, Healthcare, Packaging, Printing, Textiles |
Market Drivers | Oil & gas, paints & coatings, plastics, paper, medical imaging |
Note: Precipitated barium sulphate dominates high-end applications, such as advanced coatings, electronics, and pharmaceuticals. Natural barium sulphate remains essential for bulk fillers and cost-driven sectors. Both types contribute to the development of radiation shielding materials and other specialized products.
Natural barium sulphate and precipitated barium sulphate show a clear price difference in the market. Natural barium sulphate uses a straightforward production process. Workers mine, crush, and separate the ore. This method gives a high yield and keeps costs low. The market price for natural barium sulphate stays around CNY 1,500 per ton. Precipitated barium sulphate, on the other hand, needs a complex chemical process. It involves several steps such as calcination, synthesis, extraction, precipitation, and flash evaporation. Each step uses more raw materials and energy. Fewer companies produce precipitated barium sulphate because of these high costs. As a result, its market price is much higher than that of natural barium sulphate.
Type | Typical Production Process | Market Price (per ton) |
|---|---|---|
Natural Barium Sulphate | Mining, crushing, separation | ~CNY 1,500 |
Precipitated Barium Sulphate | Chemical synthesis, precipitation | Significantly higher |
Tip: Buyers should compare both the initial cost and the performance benefits before making a decision.
Several economic factors affect the value of both types of barium sulphate. The cost of precipitated barium sulphate depends on raw material prices, labor costs, and energy use. Inflation and exchange rates also play a role in setting prices. Regional economic growth can change demand and supply, which impacts pricing. Natural barium sulphate benefits from stable production and a larger supply base. This stability helps keep its price lower and more predictable. Precipitated barium sulphate faces more price swings because of its complex process and higher input costs. Companies must weigh these factors when choosing which type to use in their products.
Natural barium sulphate offers a cost-effective solution for industries that need large volumes.
Precipitated barium sulphate delivers higher purity and performance, but at a premium price.
Market trends show that economic shifts can quickly change the cost structure for both types.
Note: Understanding these economic factors helps buyers make informed choices and manage their budgets more effectively.
Selecting the right type of barium sulphate depends on several important factors. Each industry and application has unique needs. Companies must consider these points before making a decision:
Factor | Natural Barium Sulphate | Precipitated Barium Sulphate |
|---|---|---|
Purity Needs | Moderate purity, less control | High purity, strict control |
Particle Size | Larger, less uniform | Finer, highly uniform |
Cost Constraints | Lower cost, bulk supply | Higher cost, premium applications |
Application Type | Drilling, plastics, bulk fillers | Medical, coatings, electronics |
Regulatory Standards | Fewer restrictions | Meets strict regulations |
Regional Availability | Widely available | Limited, specialized suppliers |
Medical imaging and pharmaceutical industries require high purity and consistent particle size. Precipitated barium sulphate fits these needs best.
Oil and gas drilling, plastics, and construction often use natural barium sulphate. It offers a cost-effective solution for large-scale projects.
Technological advances, such as nano-sized particles, improve performance in coatings and medical imaging. Companies may choose specialized grades for these uses.
Regional market trends and regulations also influence the choice. For example, Asia-Pacific industries may focus on cost, while Europe demands eco-friendly, high-purity products.
Tip: Companies should match the grade of barium sulphate to the specific requirements of their industry and application.
Natural barium sulphate offers a cost-effective choice for bulk uses, while precipitated barium sulphate provides high purity and fine particles for demanding industries. Buyers should compare their technical needs and budgets before choosing.
Use natural barium sulphate for drilling or plastics.
Select precipitated grades for medical or advanced coatings.
For best results, consult suppliers or industry experts. They can help match the right product to each application.
Natural barium sulphate comes from barite ore. It contains more impurities and larger particles. Precipitated barium sulphate forms through a chemical process. It offers higher purity and finer, more uniform particles.
They select precipitated barium sulphate because it meets strict purity and particle size standards. These qualities ensure safety and effectiveness in medical imaging and pharmaceuticals.
No. Natural barium sulphate works well in cost-sensitive, bulk uses like drilling fluids. It cannot match the purity or performance needed for advanced coatings or medical products.
Smaller, uniform particles improve strength, appearance, and stability in paints, plastics, and medical uses. Larger, uneven particles may weaken materials or reduce effectiveness.
Yes. The chemical process for precipitated barium sulphate costs more. It uses extra steps and energy. This makes it more expensive than natural barium sulphate.
Copyright 2024 GUANGZHOU TIPTOP NEW MATERIAL CO., LTD. Sitemap

